Category: Regulations
-
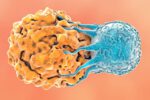
Trial Okayed for Engineered Cell Cancer Therapy
A clinical trial is set to begin testing engineered immune-system cells designed to precisely target and infiltrate cancerous tumors without other potentially toxic proteins.
-

FDA Issues Action Plan for Rare Neuro Diseases
The Food and Drug Administration published its blueprint to encourage new treatments for rare neurodegenerative disorders, including ALS.
-
Trial Set for Gut Infection Biologic Treatment
A developer of biologic therapies for gastrointestinal disorders says FDA cleared its request for a clinical trial testing its treatment for C. difficile.
-
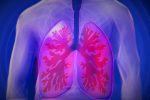
Trial Set for Respiratory Disease Nasal Spray Vaccine
A clinical trial is about to begin enrolling participants to test a vaccine given as a nasal spray protecting against respiratory syncytial virus, or RSV.
-

Precision Meds Continue Gaining FDA Approvals
Food and Drug Administration approved 17 new medications in 2021 addressing genetic targets, according to data from a precision medicine advocacy group.
-
FDA Issues Guidance for Non-Opioid Acute Pain Relief
The Food and Drug Administration released for comment review processes for new non-opioid drugs to relieve acute or temporary pain episodes.
-

India Authorizes Covid-19 Vaccines Designed in US
The government of India granted emergency clearance to two vaccines to prevent Covid-19 disease, both designed in U.S. research labs.
-

FDA Clears Rapid At-Home Covid-19 Test
The Food and Drug Administration granted an emergency authorization to a quick diagnostic test for SARS-CoV-2 viruses, conducted at home.
-

Essay – Quick, Name the Secretary of HHS
Our country and world are in the worst health crisis in a century, and the U.S. cabinet secretary with the word “health” in the title is missing in action.
-
Covid-19 Antibody Cocktail Trial Request Filed
A discoverer of synthetic antibodies is asking Food and Drug Administration for clearance to begin a clinical trial of a Covid-19 antibody cocktail therapy.